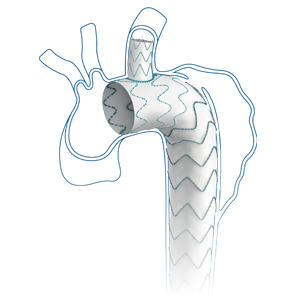
Castor™ Branched Stent-Graft
Furthering Treatment for Advance Pathologies
- Multi-Dimensional Angles
Allow orientation of the branch to various LSA anatomy requirements. - Unique Unibody Design
Reduces the risk of Type III endoleaks and migration.
Cratos™ Branched Aortic Stent-Graft System, custom made device.
Cratos™ is an upgraded product launched by Endovastec™ based on the Castor™ Branched Stent-Graft. The Castor™ Branched Stent-Graft, as the world's first approved branched aortic stent graft, was launched in China in 2017, received CMD custom permission in Europe in 2022, and has entered clinical use in 18 countries globally, successfully treating over 20,000 patients worldwide. It has become the preferred device for treating Stanford Type B aortic dissections involving the arch branches.
In terms of stent graft, Cratos™ inherits the integrated branch structure design of the Castor™ Branched Stent-Graft, significantly expanding the stent landing zone while effectively reconstructing the LSA. In terms of its delivery system, Cratos™ has undergone several innovative upgrades: employing a new inner curvature control release technique; integrating an active stent proximal adjustability feature (enhancing the accuracy of stent positioning and eliminating the “bird-beak” at the stent proximal end for better adherence); reducing the sheath diameter of the stent system to 22F (further lowering the requirements for the access vessels, thereby expanding the range of application); and incorporating a longer sheath, rotating plus quick-release method, and misuse-proof design, simplifying the surgical steps.